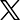Research Update: Pioneering Clinical Trial Gets Underway
amfAR-funded study will test combination cure strategy

A unique and potentially groundbreaking clinical trial led by researchers at the amfAR Institute for HIV Cure Research is underway. The first participant received the first injection on August 13. The study will test a combination of agents in an effort to induce post-treatment control in people living with HIV.
The complex, multi-stage trial is the culmination of four years of work by teams of researchers at the Institute, which amfAR launched in 2016 with a five-year $20
million grant to the University of California, San Francisco (UCSF).
A Combination of Agents
“The Institute will leverage three highly complementary strategies that appeared to induce remission in previous studies,” said Dr. Rowena Johnston, amfAR Vice President and Director of Research.
“It’s by far the most complex cure trial that anyone has undertaken to date and it reflects our expectation that, like the drug cocktails we use to treat HIV, a cure or post-treatment control of the virus is most likely to result from a combination of agents.”
Led by veteran HIV researcher Dr. Steven Deeks, the trial will enroll up to 20 people living with HIV who have been on stable, continuous antiretroviral therapy (ART) for at least one year. The investigators plan to include 15 participants who started ART during the early stage of infection, and five who started after a longer period of infection, to assess differences in the immune response between the two groups.
The centerpiece of the strategy is to boost the immune response, with a focus on CD8 T cells, or killer T cells. “The advantage of CD8 T cells is they can recognize infected cells specifically, and they can actually kill them,” said Dr. Rachel Rutishauser, an immunologist and researcher at UCSF. “The purpose of the first few stages [of the trial] is to elicit that initial CD8 T cell response.”
Stages 1-2: Vaccine and Boost
In the first two stages of the trial, lasting 24 weeks, participants will receive a therapeutic vaccine with a boost. The DNA plasmid vaccine is effective against almost all subtypes of HIV seen around the world. “The strategy here is to go after regions of the virus that can’t escape from the immune system,” said Dr. Rutishauser.
The boost, along with an additional adjuvant—both commonly used in vaccine trials—will help to enhance the immune response.
“It’s by far the most complex cure trial that anyone has undertaken to date.”
Stage 3: Coax and Activate
The third stage will involve the administration of two additional agents. The first is a TLR9 agonist, which the researchers hope will coax the virus out of latently infected reservoir cells so that it can be detected by the immune system. As an immune adjuvant, it will also further boost the immune system, and researchers think that it will help natural killer cells of the innate immune response kill infected cells.
Participants will also receive broadly neutralizing antibodies that can inactivate diverse types of HIV and reduce the size of the viral reservoir. There is also compelling evidence from animal studies that these specialized antibodies enhance the CD8 T cell response.
Stage 4: Interrupt Treatment

In the fourth and final stage of the trial, at 34 weeks, participants will undergo an analytic treatment interruption to test the efficacy of the combination approach. At the same time they stop taking ART, participants will be given a second round of broadly neutralizing antibodies. As the initial dose of antibodies wears off, this step is meant to help the immune response outpace the virus as it emerges from latency.
Researchers will assess whether post-treatment control has been achieved by measuring the proportion of participants that show sustained viral suppression after treatment interruption. The safety of the combination approach will be gauged by closely monitoring for adverse events.
There will also be a behavioral component to the trial. Members of amfAR’s Community Advisory Board are making active contributions, which include assessing participants’ understanding of the study, their comfort level with the combination of interventions, and their experience throughout the process.
“Naturally we hope this trial succeeds in achieving post-treatment control,” said Kevin Robert Frost, amfAR’s Chief Executive Officer. “But whatever the outcome, the trial will generate invaluable new knowledge that is sure to advance our search for a cure for HIV.”
Share This: